Antibiotic sensitivity testing transformed infectious disease care. Pharmacogenomics could do the same for everyday prescribing, but only with the right infrastructure.
Pharmacogenomics is the study of how genetic variation affects drug response in people. Through this, the objective is to optimize drug prescription in order to have maximum efficiency and minimal side effects. While the academic field of pharmacogenomics has evolved and produced results, most healthcare technology today is not ready to incorporate these insights. In an episode of the Digital Health Hackers podcast, Dr. Sidharth Ramesh discusses this and more with Dr. Videha Sharma.
Dr. Videha Sharma is the Clinical Innovation Lead at the Health Technology Institute, University of Manchester. He is also the CEO of Fava Health, whose mission is to integrate genomics insights into existing clinical workflows with technology solutions. Before he pivoted to health IT, he was a frontline kidney transplant physician with over 12 years of clinical expertise.
In this episode, Videha shares his journey from medicine to digital health, driven by a need to scale healthcare impact with better design and infrastructure.
A clinician’s journey into health informatics

Videha’s journey into healthcare was driven by deeply personal experiences. Growing up in Rotterdam, his younger brother had autism and severe learning disabilities. The interventions by healthcare and social care professionals had a profound impact on their family, and left a lasting impression. “It really shaped my desire to want to pursue a career in healthcare, and really have an impact.”
He trained in the UK, eventually specialising in kidney transplantation. “For me, it was the best job in the world. I loved turning up early, staying late, doing procedures, and looking after patients.” Twelve years later, he decided to pivot to healthcare. This shift didn’t come from dissatisfaction, but he puts it as wanting to have “impact at scale”.
Videha went on to do a PhD in Health Informatics. This pivot echoes Sidharth’s own journey, where he realized that working with healthcare data could have an impact on the health outcomes of many patients at once. Now, Videha works with healthcare providers, industry partners and academia to translate emerging technologies like pharmacogenomics into real clinical practice.
Healthcare needs a design-led, creative mindset
A significant difference Videha noticed since moving away from clinical practice, is a difference in thinking. Doctors are used to thinking convergently, “As soon as the patient walks in, you go from a long list of potential problems to a short list of differential diagnoses.” Convergent thinking is necessary in time-pressured situations which is often the case for consultations.
When tackling challenges like hospital waiting times, workforce shortages, or technology adoption, Videha emphasizes the need for divergent thinking. Design thinking approaches have a role to play here, in understanding the problem before jumping into solutions, involving people affected by the problem, and bringing in expertise beyond medicine.
Personalized medicine with pharmacogenomics
Videha talks about how pharmacogenomics is a way in which healthcare can work better. Generic variation affects how patients respond to many commonly prescribed medications, including antidepressants, statins, opiate analgesics, and antiplatelet drugs. Today, prescribing these drugs follows a costly trial-and-error approach, leading to delayed treatments and adverse effects for patients.
“Besides causing delays in receiving effective treatment, it also has a significant impact on side effects and adverse drug reactions,” Videha says. In the UK alone, more than one in twenty hospital admissions are related to adverse effects. The core question Videha poses is simple: “What if patients and doctors knew what medicines are most safe and effective from the start?”
The main challenge with getting this information available at the right time is with implementation, and integration with existing clinical workflows.
Incorporating pharmacogenomics into real workflows
At Fava Health, Videha and his team work on closing this implementation gap. For an end-to-end pharmacogenomics service, they envision a system in three parts:
- Testing: Implementing and tracking logistics around genomics testing and associated technologies
- Digital data platforms: Analysing, summarizing and standardizing of genomics results, and doing this using open standards
- Reporting: Ensuring clinician-facing decision support, patient access to data and reuse of data for research
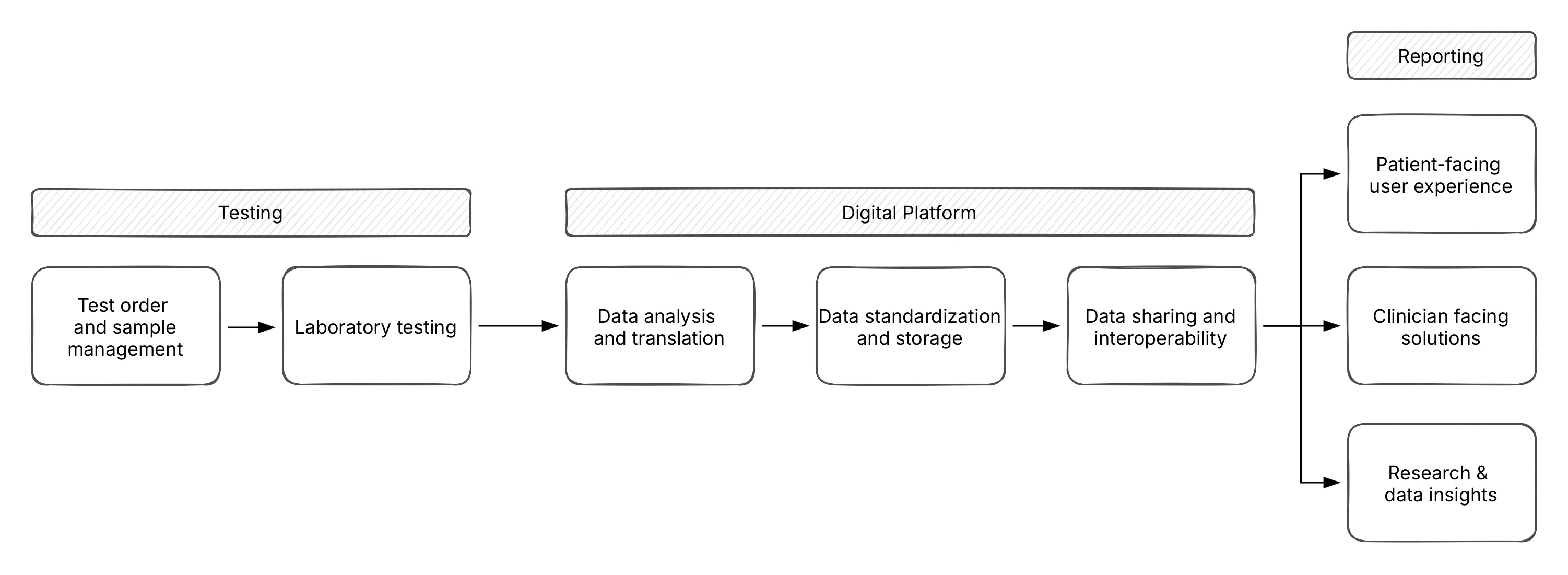
What makes this approach different is the focus on use and not theory. When working across academia, industry and technology, Videha and the team at Fava health ensure that each decision caters to the requirements of clinicians. Real value lies in embedding results into the EHR workflow, he says.
Genetic results can also be complex and difficult to interpret, and so translation is a part of this infrastructure. “Clinicians don’t have the time to understand CYP2D6 intermediate metabolizers. They need simple, actionable language.” Videha works with the Global Alliance for Genomics and Health to ensure that standards for representing pharmacogenomics results are suitable for clinical environments. What is needed to make pharmacogenomics mainstream? Even though there is strong clinical evidence, Videha describes that it is economic viability that will make pharmacogenomics more accessible in the mainstream.
Significant upfront capital investment is required to perform genomic testing at scale. This includes sequencing machines that can cost close to a million dollars, which can be particularly challenging for single payer systems like the NHS. There are also practical questions about where and how testing happens. Private genomic testing is growing, but clinicians struggle to trust and integrate external test results into care systems.
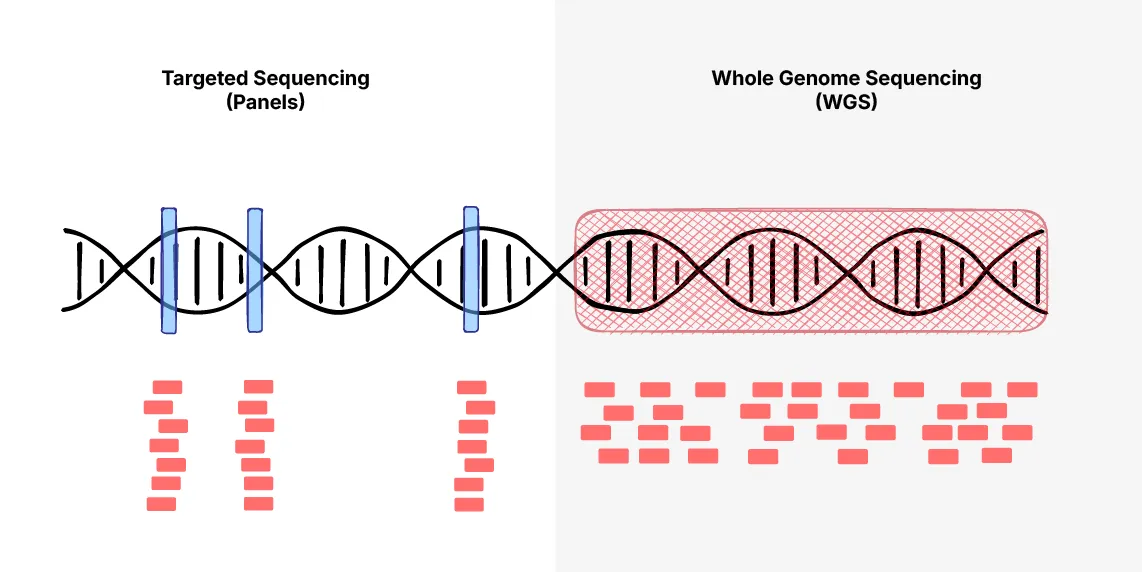
On whole genome sequencing, Videha suggests caution, “The majority of information in our genetic code, we don’t know what it means yet.” His stance is guided by strong data governance principles, to only collect data with a clear, intended use. So, in the near term, targeted gene panels would be more responsible, and practical.
Integrating genomics data presents an opportunity for health IT infrastructure. “Because this data isn’t locked in legacy EHRs, we can design it properly from the start.” They have a firm architectural position, to store genomic data external to EHR systems, in vendor neutral repositories that are accessible through APIs. This would also serve to decouple data from applications in order to survive system changes.
Closing thoughts
The future of personalized medicine through pharmacogenomics holds significant promise. As Dr. Videha Sharma points out, the key to this progress lies in the infrastructure to integrate these technologies into everyday clinical workflows. Embedding genetic results into existing EHR systems and offering interpretable insights to physicians is the key to making it accessible and scalable.
This infrastructure that Videha and his team at Fava health envisions relies on storing data in standard, vendor-neutral repositories that integrate smoothly with existing systems. The translation of academia-oriented generic results into actionable, simple insights for clinicians is another aspect that they work with.
However, economic feasibility is key. The upfront costs can be prohibitive to many single-payer systems, and can delay adoption. Reliability and accreditation of private testing services can also play a role here. As we move towards a data-driven and patient-centric future, we see how existing gaps in technology and infrastructure become barriers to affordable personalized healthcare. The path forward requires collaboration, innovation and a focus on putting clinician-needs first when designing these solutions.
Watch the complete interview, Bridging Genes and Digital Health (DHH Podcast Ep. 4), and check out our free fundamentals courses if you’re interested in learning more about open standards like FHIR and openEHR.